What Pairs of Elements Form Ionic Compounds
Same reason as above. 3- fluorine and sodium.
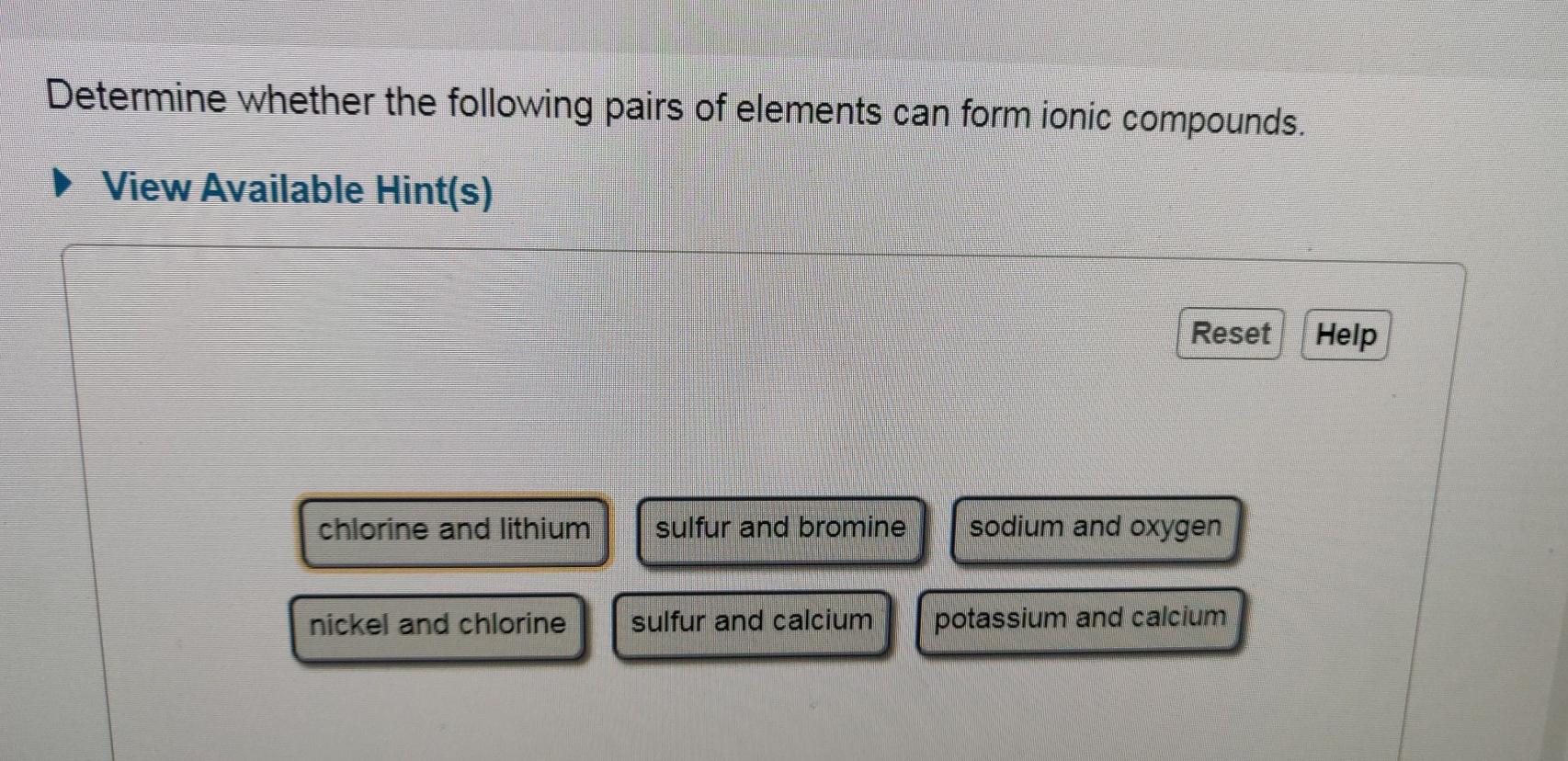
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com
D potassium and sulfur.
. Na and Cl 2. Please not the attached picture is not the correct answer. In option E barium is a metal forms cation and bromine is a non.
615 which of the following pairs of elements are likely to form an ionic compound. Forms ionic empirical formula of ionic compound element 1 element 2 compound. Cesium and magnesium f.
Subsequently one may also ask which pair of elements will form an ionic compound. Potassium and sulfur helium and oxygen magnesium and chlorine sodium and potassium nitrogen and iodine chlorine and bromine. Step 1 of 4.
Cesium fluorine O yes O no do sodium iodine O yes O no Ar barium cesium O yes O no oxygen. In this compound there are two negative chloride ions for each positive calcium ion. Identify the kinds of ions that form each ionic compound.
When a metal and a nonmetal exchange electrons to make a cation and an anion respectively they can form an ionic compound. Forms ionic bonds because one of the elements is metal and the other is not metal. Potassium and oxygen d.
The following pairs of elements react to form ionic compounds. A Sodium and Magnesium b Oxygen and Magnesium c Fluorine and Sodium. Aluminum bromide AlBr3 c.
11 Which pair of elements is most apt to form an ionic compound with each other. Lithium oxide Li2O d. Write the chemical formula and chemical name of the ionic compounds formed.
Ca and Cl S and K Option A and B. Determine whether the following pairs of elements can form ionic compounds. 15 Which element does not usually form compounds.
A Sodium and Magnesium b Oxygen and Magnesium c Fluorine and Sodium d Sulfur and Bromine e Manganese and Chlorine f Potassium and Sulfur. Usually a metallic element and a non metallic element form an ionic compound but. A helium and oxygen.
Hence lithium and bromine pair of. Ionic bonds usually occur between metal and nonmetal ions. Following are the pair of elements that result in an ionic compound.
Which of the following pairs of elements are likely to form ionic compounds. Decide whether each pair of elements in the table below will form an ionic compound. Determine whether the following pairs of elements can form ionic compounds.
For example sodium Na a metal and chloride Cl a nonmetal form an ionic bond to make NaCl. 16 Which of the elements do not form compounds easily and why. Magnesium and chlorine will form an ionic compound.
2- oxygen and magnesium. 17 What I have learned about elements and compounds. A fluorine and sodium B oxygen and magnesium C potassium and sulfur D iron and chlorine E.
Which pair of elements form an ionic compound. Ionic compounds generally form between elements that are metals and elements that are nonmetals. B magnesium and chlorine.
Determine whether the following pairs of elements can form ionic compounds. F nitrogen and oxygen. These compounds have a positive charge of ions called cations and a negatively charged element is anions.
View the full answer. Sodium and magnesium nitrogen and bromine. Lithium is a metal on the left side of the periodic table transfers one electron to bromine which is a nonmetal on the right side of the periodic table and forms an ionic bond between lithium and bromine.
In the above options we. If they will write the empirical formula of the compound formed in the space provided. The elemental pairs that form ionic compounds are Sodium and Oxygen Manganese and Chlorine Oxygen and.
Which of the following pairs of elements are likely to form ionic compounds. Which pair of element can combine to form ionic bonds. For example the metal calcium Ca and the nonmetal chlorine Cl form the ionic compound calcium chloride CaCl 2.
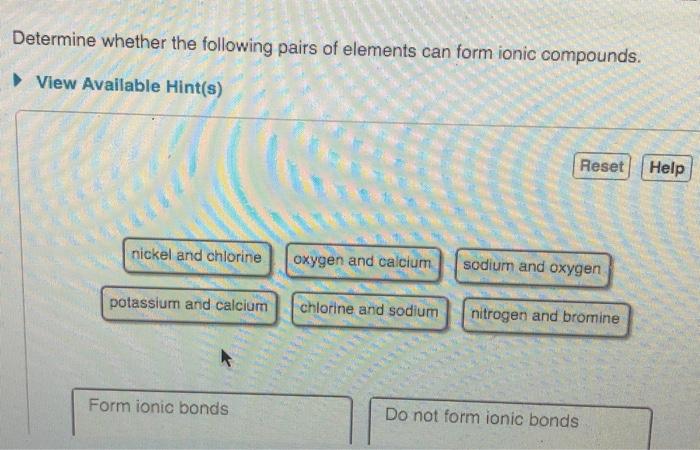
For example the metal calcium Ca and the nonmetal chlorine Cl form the ionic compound calcium chloride CaCl 2. Fluorine and sodium nickel and chlorine potassium and sulfur oxygen and calcium Do not form ionic bonds. Determine whether the following pairs of elements can form ionic compounds.
A Ionic compounds are formed by the transfer of electrons between the metals on the left side and the nonmetals on the right side. 1- iron and cholrine. A barium bromine B calcium sodium C oxygen fluorine D sulfur fluorine E nitrogen hydrogen.
Na and O 2. Forms ionic bonds because one of the elements is metal and the other is not metal. Ionic compounds generally form between elements that are metals and elements that are nonmetals.
Sodium and potassium wont form an ionic compound. In a covalent bond the atoms bond by sharing electrons. Lithium and chloride b.
Ionic compounds are defined as the compound that consists of ions held by two atoms of differently charged ions. 18 What is the characteristic of elements and compounds. These are two metals and ionic compounds are formed between a metal and a non-metal.
Sodium and neon e. The ionic bond formation takes place when electrons get transferred to a non-metal from a metal. Determine whether the following pairs of elements can form ionic compounds.
19 What are some facts about elements and compounds. Calcium fluoride CaF2 b. Ionic compounds are formed betweena metal and a non-metal.
Forms ionic bonds because one of the elements is metal and the other is not metal. 20 When elements form compounds the elements react to form a new. Ionic Compounds involves strong electrostatic forces.
C chlorine and bromine. Aluminum sulfide Al2S3 e. That is polar compounds usually are soluble in water and non-polar.
E sodium and potassium. - sodium and oxygen. ALi and S BO and S CAl and O dF and Cl eI and K FH and N Which of the following pairs of elements will not form ionic Chemistry - solubility Arrange the following compounds in order of increasing solubility in water.
Oxygen and bromine c. A fluorine and sodium B oxygen and magnesium C potassium and sulfur D iron and chlorine E nitrogen and iodine F lithium and calcium. Covalent bonds usually occur between nonmetals.
Did this page answer your question. O2 LiCl Br2 CH3OH Like dissolves like. Potassium and sulfur wont form an ionic compound.
View Available Hint s Reset Help nuorine and sodium DO Part A Determine whether the following pairs of elements can form ionic compounds View Available Hint s Reset Help potassium and.

Which Pair Of Elements Would Form An Ionic Bond A Carbon C And Oxygen O B Strontium Sr Brainly Com

Oneclass Please Answer It Which Of The Following Pairs Of Elements Are Likely To Form Ionic Compound
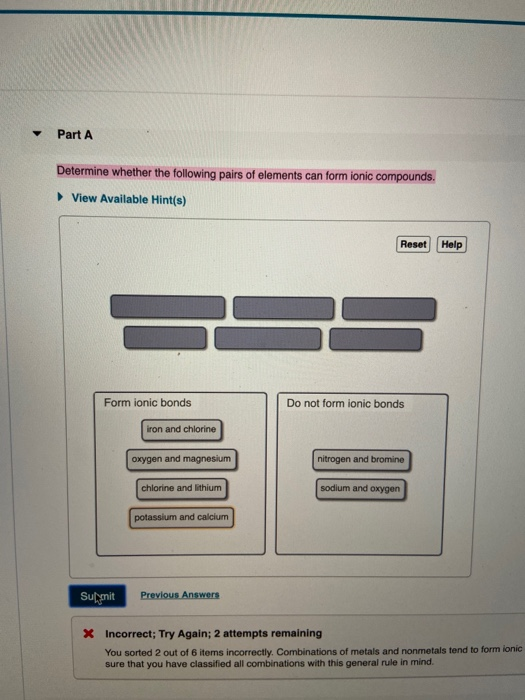
Solved Part A Determine Whether The Following Pairs Of Chegg Com
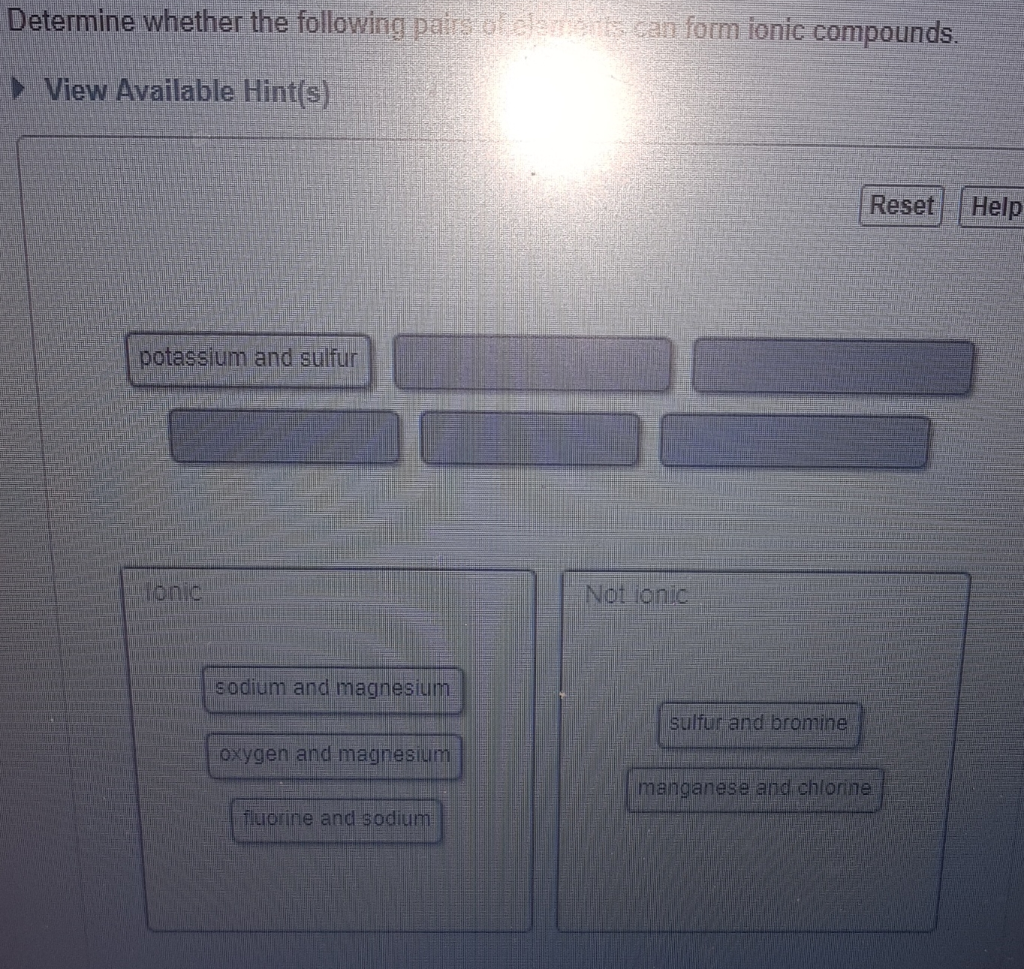
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com
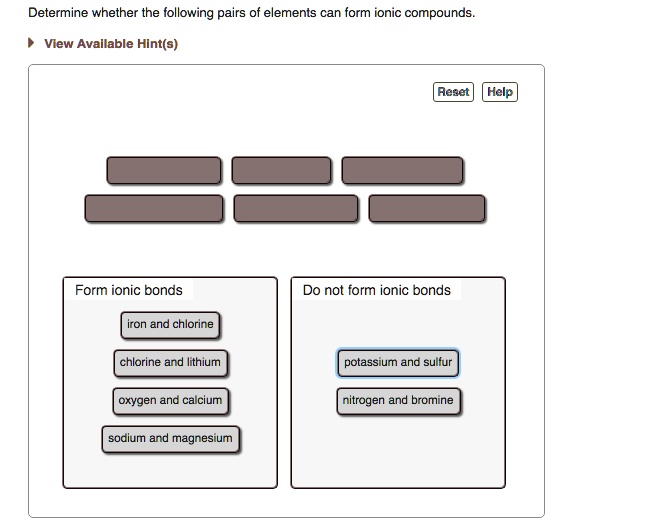
Solved Determine Whether The Following Pairs Of Elements Can Form Ionic Compounds Vlew Avallable Hint S Aeset Help Form Ionic Bonds Do Not Form Ionic Bonds Iron And Chlorine Chlorine And Iithium Potassium And

Get Answer Determine Whether The Following Pairs Of Elements Can Form Transtutors

Which Of The Following Pairs Of Elements Could React To Form An Ionic Compound Check All That Apply Brainly Com

Which Of These Pairs Of Elements Would Be Most Likely To Form An Ionic Compound A Ca And Br B C And O C P And Cl D Al And K

Solved Determine Whether The Following Pairs Of Elements Can Chegg Com
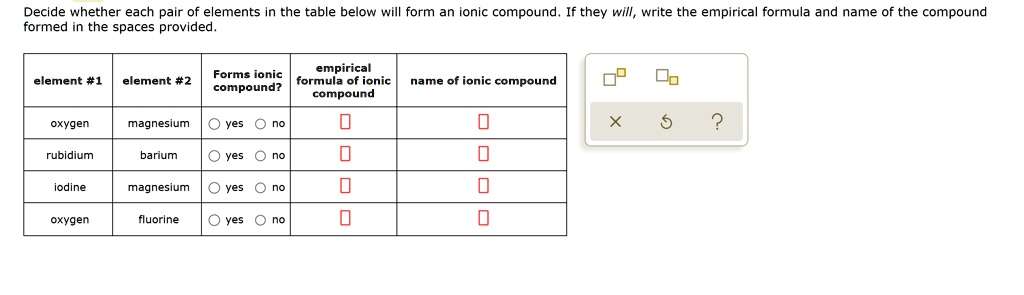
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Onic Compound Formed In The Spaces Provided They Will Write The Empirical Formula And Name Of The Compound Empirical

Aleks Predicting And Naming Ionic Compounds Formed By Two Elements Youtube
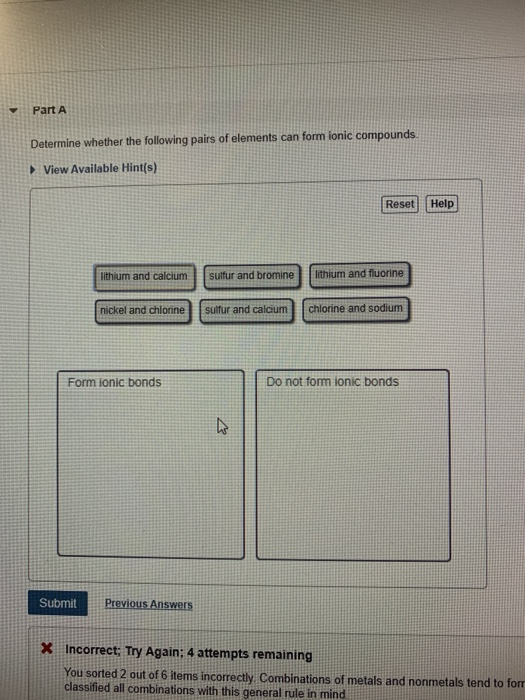
Solved Part A Determine Whether The Following Pairs Of Chegg Com

Ionic And Metallic Bonding Ppt Video Online Download
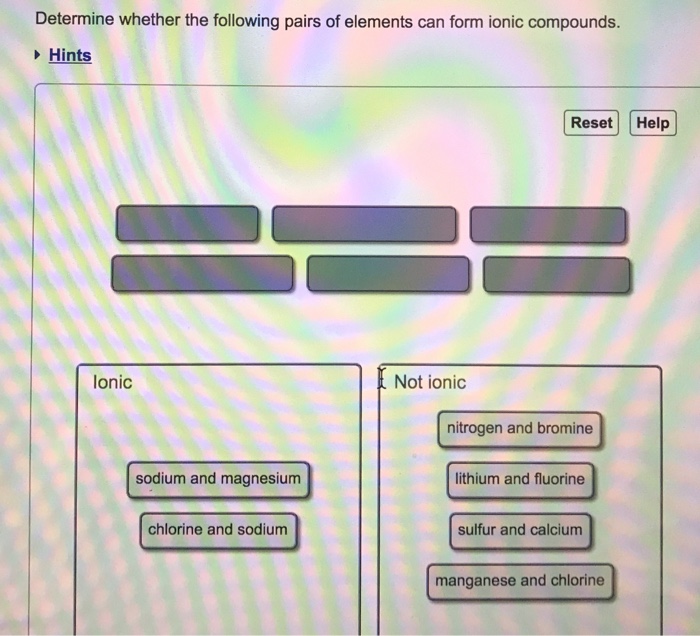
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com

Determine Whether The Following Pairs Of Elements Can Form Ionic Compounds Brainly Com
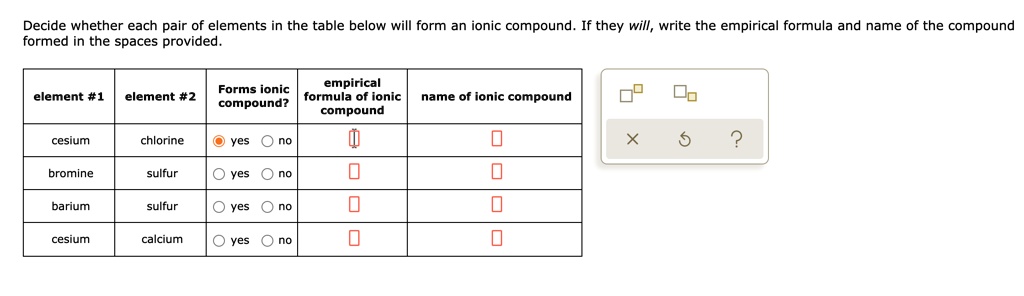
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula And Name Of The Compound Formed In The Spaces Provided
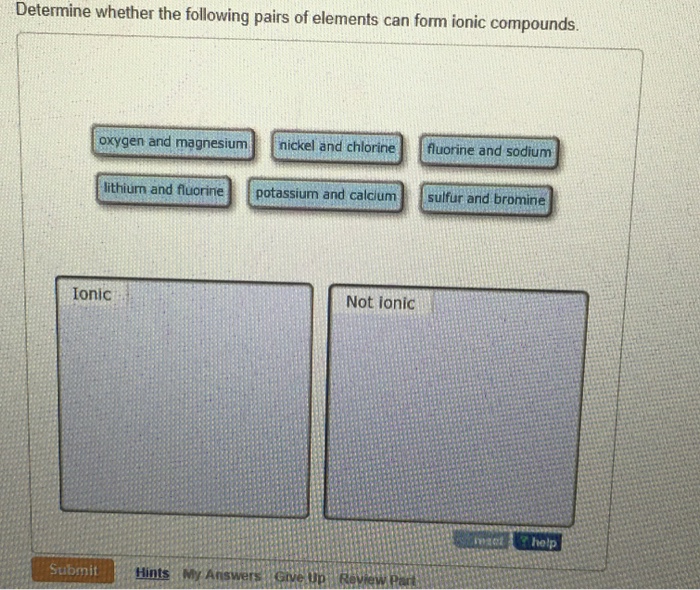
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com

Atoms Of Which Pair Of Elements Will Form Ionic Bonds In A Compounda I And Fb Ag And Cac Be And Brainly In

Comments
Post a Comment